
- CATHODE RAY EXPERIMENT CONCLUSIONS PATCH
- CATHODE RAY EXPERIMENT CONCLUSIONS FULL
- CATHODE RAY EXPERIMENT CONCLUSIONS SERIES
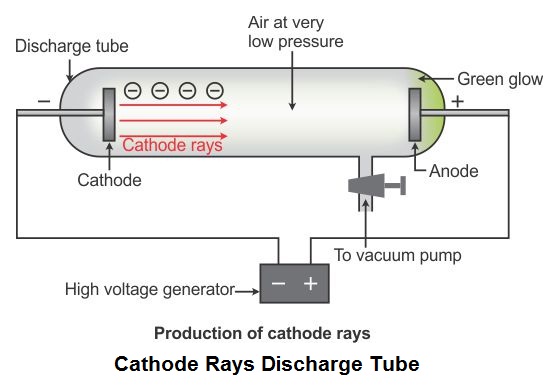
This cookie is set by GDPR Cookie Consent plugin. These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. It was highly likely for them to be one of the components of atoms. Jun 1 2019īy using certain modifications in the regular CRT, Thomson ’s cathode ray tube experiment proved that cathode rays consist of streams of negatively charged particles having smaller masses than that of atoms. The cathode ray is drawn to the positively charged plate, called the anode, where it passes through a hole and continues travelling to the other end of the tube. When the two metal plates are connected to a high voltage source, the negatively charged plate called cathode, emits an invisible ray.
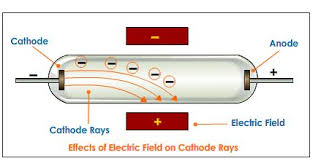
What are the principles of cathode ray tube? He concluded that the negative charge and the rays were one and the same. Thomson observed that the electrometer registered a charge only when he deflected the cathode ray to it with a magnet.
CATHODE RAY EXPERIMENT CONCLUSIONS PATCH
Thomson could trace the path of the ray by observing the phosphorescent patch it created where it hit the surface of the tube. What did Thomson conclude about cathode rays? Thomson interpreted the deflection of the rays by electrically charged plates and magnets as evidence of “bodies much smaller than atoms” (electrons) that he calculated as having a very large value for the charge-to-mass ratio.
CATHODE RAY EXPERIMENT CONCLUSIONS SERIES
J.) Thomson (1856-1940) performed a series of experiments in 1897 designed to study the nature of electric discharge in a high-vacuum cathode-ray tube, an area being investigated by many scientists at the time. Who conducted experiments with cathode ray tubes? Rutherford’s gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Thomson’s experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. This theory further helped physicists in understanding the structure of an atom. Thomson concluded that rays were and are basically negatively charged particles present or moving around in a set of a positive charge. Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged “soup.” What was one conclusion of the cathode ray tube experiment?Ĭonclusion. What was the major conclusion of the cathode ray experiment? Thomson invented to test the theory that negative charges in an atom were real. What is the cathode ray tube? The cathode ray tube was what J.J. Thomson is the scientist that discovered electrons through an experiment called the Cathode Ray Experiment. What was discovered in the cathode ray tube experiment quizlet? Thomson’s plum pudding model of the atom had negatively-charged electrons embedded within a positively-charged “soup.” Thomson’s plum pudding model of the atom had negatively-charged electrons embedded within a positively-charged “soup.” What was the importance of J.J. Thomson (1856–1940) performed a series of experiments in 1897 designed to study the nature of electric discharge in a high-vacuum cathode-ray tube, an area being investigated by many scientists at the time. What device did Thomson use in his experiments? Corpuscles are much smaller than atom itself. What was Thomson�s conclusion from cathode ray tube experiments? All atoms contain negatively charged particles, which he named as ‘corpuscles’. What were three major conclusions of Thomson’s cathode ray tube experiment?
CATHODE RAY EXPERIMENT CONCLUSIONS FULL
the rule that states that atoms tend to be the most stable when their valence shells are completely full normally, when the valence shell contains eight valence electrons.

Thomson’s cathode ray tube experiments provided the first evidence that atoms were composed of even smaller particles called electrons. Thomson’s cathode ray tube experiment show quizlet? Thomson used the cathode ray tube to determine that atoms had small negatively charged particles inside of them, which he called “electrons.” What did J.J. Electrons were first discovered as the constituents of cathode rays. How did Thomson use the cathode ray tube?Ĭathode rays carry electronic currents through the tube. 7 What are the principles of cathode ray tube?.5 What was one conclusion of the cathode ray tube experiment?.1 How did Thomson use the cathode ray tube?.


 0 kommentar(er)
0 kommentar(er)
